Thermodynamics has its own basic concepts. It is very important to understand these terms of thermodynamics to avoid any kind of confusion. It deals with various kinds of terms like system, system boundary, surroundings, etc. So, we will discuss all these terms one by one in detail.
Table of Contents
“System” In Thermodynamics:
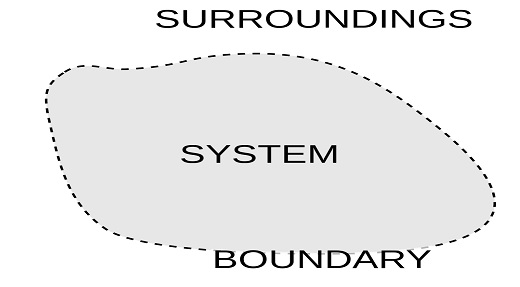
In thermodynamics, a system is defined as the particular area or region having energy or matter which is separated from the surrounding by a wall or a boundary. The boundary separating the system from the surroundings can real or imaginary. So, from this, we can understand that a thermodynamic system is a particular area of matter with a defined boundary on which our focus lies.
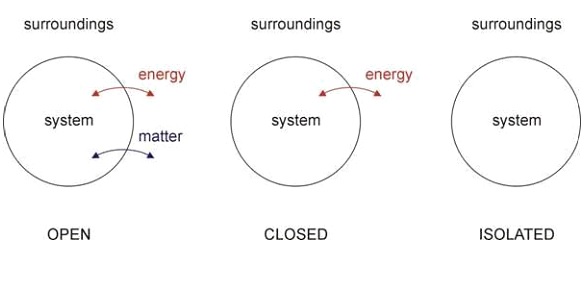
Different Kinds Of Thermodynamic Systems:
Isolated System:
In an isolated thermodynamic system, heat, work, and mass flow are not possible. From the name itself you can understand that it is isolated from its surrounding. So, no exchange will happen between the isolated system and its surrounding. The universe is an example of an isolated system.
Closed System:
In a closed thermodynamic system, the exchange of energy can happen with its surrounding. That means work and heat transfer are possible in a closed system, but the mass flow is not possible in this case. The refrigerator is a good example of a close thermodynamic system.
Open System:
From the name itself, you can get the idea that it is an open system. That means in the open thermodynamic system both energy as well as mass can be transferred. So, in this case, work, heat, and mass flow exchange can happen with the surrounding.
“Surroundings” In Thermodynamics
Surroundings in thermodynamics can be defined as everything other than the system itself which can directly affect the system behavior. So, in simple words, we can say that everything except the system is surrounding which interacts with it. For example, if you consider your body as a system, then except your body whole universe is surrounding which interacts with it.
“System Boundary” In Thermodynamics
System Boundary in thermodynamics can be defined as a closed surface area surrounding the system through which all the interaction takes place. Through a system boundary only, the energy and mass flow happen. So, in an open thermodynamic system, if work, heat, and mass are transferred or exchanged with the surroundings, it will only happen through the system boundary. That’s why the boundary of a system in thermodynamics is a very important place for the exchange of energy and mass.
